By Normand Brais, P.Eng., M.A.Sc., Ph.D. and Benoit Despatis, Eng. ASHRAE Member
It has been often noticed by many users over the years that whenever a germicidal UV surface disinfection is performed in a room, there is almost always a strange odor left afterward. It is not the smell of ozone, which can be easily identified and measured. It is more like a slightly pungent smell similar to rotten eggs or burnt hair. It is actually easier to recognize the smell than to describe it. Up to now, no satisfactory explanation as to the origin of this peculiar odor has been provided. Several working hypothesis have been explored to explain this awkward phenomena:
1) Off-gassing of wall surfaces such as paint or other volatile materials.
2) UV lamps end caps glue off-gassing.
3) UV lamps connectors or end rubber boots overheating.
4) Interaction of UV with airborne and surface-borne dust.
After several tests and experiments, the first three hypotheses were quickly ruled out as a potential root cause. Off-gassing of paint was eliminated after testing in a bare metal aluminum enclosure and witnessing the same odor.
The UV lamps end caps were completely removed and all the glue removed with no effect. The same was done for the lamps connectors and also showed no impact on the odor. However, while we were performing these tests, it was noticed that when the disinfection cycles were repeated several times in the same enclosure, the perceived odor level after each cycle seemed to be diminishing. This was the hint that leads us to focus our attention on the presence of dust particles in the air, what these particles consist of, and how UV can potentially alter them into perceptible odorous compounds.
Airborne dust in homes, offices, and other human environments typically contains up to 80% of dead human skin and squamous hair, the rest consists of small amounts of pollen, textile fibers, paper fibers, minerals from outdoor soil, and many other micron size materials which may be found in the local environment1,2. In a typical indoor environment, the airborne dust volumetric load is somewhere between 100 and 10,000 μg/m3 (0.000044 to 0.0044 grain/ft3) order of magnitude. The dust load depends upon the occupancy rate, type of human activity, air filtration system efficiency, etc. It is worth noting that the maximum acceptable ASHRAE level for total dust is 10,000 μg/m3 (0.0044 grain/ft3) and 3,000 μg/m3 (0.0013 grain/ft3) for PM10.
Since airborne dust is essentially dead human skin and squamous hair pieces, it is worth taking a closer look at the fundamental material they are made of. The main constituent of human skin is a molecular group called keratin. Keratin is a family of fibrous structural proteins. Keratin is the key structural material making up the outer layer of human skin. It is also the key structural component of hair and nails. Keratin monomers assemble into bundles to form intermediate filaments, which are tough and insoluble. Keratins encloses large amounts of the sulfur-containing amino acid cysteine, required for the disulfide bridges that confer additional strength and rigidity by permanent, thermally stable crosslinking; a role sulfur bridges also play in vulcanized rubber. Human hair is approximately 14% cysteine. Cysteine3 is an amino acid with the chemical formula HO2CCH(NH2)CH2SH. The pungent smell of burning hair and rubber is due to the sulfur by-products. The average composition of human hair consists of 45.2 % carbon, 27.9% oxygen, 6.6% hydrogen, 15.1% nitrogen and 5.2% sulphur.4
When high energy UV-C light photons hit a keratin/cysteine molecule, they have enough power to break their internal chemical bonds and shatter them into many smaller molecules. The energy of germicidal UV photons at 254 nm wavelength is 470 kJ/mole, a value greater than the energy of chemical bonds listed in Table 1. It is therefore quite clear that proteomic molecules such as keratin and cysteine can be broken up by germicidal UV irradiation but not by visible light, for which the average wavelength is 550 nm, and the maximum photon energy only 217 kJ/mol.
Table 1. Chemical Bonds Strength5
| Chemical Bond |
Chemical Bond Average Energy |
| C – C | 347 |
| C – H | 413 |
| C – N | 305 |
| C – O | 358 |
| C – S | 259 |
| N – H | 391 |
Therefore, some of the chemical bonds between carbon atoms and hydrogen, nitrogen, oxygen and sulfur atoms will be broken by germicidal ultraviolet photons. Some of the resulting broken pieces of molecules following a sufficiently intense UV photon bombardment will contain sulfur and therefore fall into a category known as thiol molecules. Thiols are a family of sulfur compounds also called mercaptans. Their smell threshold is extremely low. The human nose can detect thiols at concentrations as low as 1 part per billion. The rotten egg-garlic smell is a dominant characteristic of mercaptans as shown in Table 2.
Burning skin emits a similar smell as thiols, while setting hair on fire produces a sulfurous odor. This is because the keratin in our hair contains large amounts of cysteine, a sulfur-containing amino acid. The smell of burnt hair can cling to nostrils for days.
Table 2. Reported Sensory Threshold for Thiol / Sulfur Compounds6
| Compound Name | Chemical Formula | Sensory Description | Smell Threshold (ppb) |
| Sulfure d’hydrogène | H2S | Œuf pourri, eaux usées | 0.5 – 1.5 |
| Éthylmercaptan | CH3CH2SH | Allumette brûlée, sulfuré, terreux | 1.1 – 1.8 |
| Méthylmercaptan | CH3SH | Chou pourri, caoutchouc brûlé | 1.5 |
| Sulfure de diéthyle | CH3CH2SCH2CH3 | Caoutchouteux | 0.9 – 1.3 |
| Sulfure de diméthyle | CH3SCH3 | Maïs en conserve, choux cuit, asperge | 17 – 25 |
| Disulfure de diéthyle | CH3CH2SSCH2CH3 | Ail, caoutchouc brûlé | 3.6 – 4.3 |
| Disulfure de diméthyle | CH3SSCH3 | Végétal, choux, oignon intense | 9.8 – 10.2 |
| Disulfure de carbone | CS2 | Sucré, éthéré, légèrement vert, sulfuré | 5 |
In order to confirm the hypothesis linking the origin of the post-UV disinfection smell to the presence of keratin and cysteine in the air dust, a straightforward molecular concentration calculation was performed.
Given the dust loading, and assuming that this dust consists of 80% skin or hair, both of these containing around 5% sulfur that will end up being broken down by UV into the smallest thiol molecules such as Methyl Mercaptan, Ethyl Mercaptan or even Hydrogen Sulfide, the concentration of Thiol can be estimated as follows:
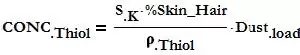
Where:
Dustload = dust weight per unit air volume in μg/m3 (lb/ft3)
SK = % Sulfur in Keratin/Cysteine = 5%
%Skin_Hair = Skin and Hair mass fraction in the dust = 80%
ρThiol = Methyl Mercaptan density at normal ambient temperature and pressure = 1.974 kg/m3 (0.1232 lb/ft3)
Equation (1) shows that when the airborne dust load gets above 75 μg/m3 (0.000033 grain/ft3), which is frequently the case in occupied spaces, the level of thiol generated by the shattering of keratin proteins exceeds the smell threshold of 0.5 to 1.5 ppb. It follows that even in the case of a relatively clean environment with dust loading as low as 100 μg/m3 (0.000044 grain/ft3), the aftermath of the UV disinfection process will leave behind a concentration of 2 parts per billion, which is greater than the smell threshold level, thus leaving behind a perceptible smell. Plotting a graph of equation 1 and allowing the dust loading to go up to 1,000 μg/m3(0.00044 grain/ft3) shows that unless the dust does not contain much dead skin or hair squames, the UV disinfection of a room will almost always leave behind a thiol concentration that exceeds the smell threshold.
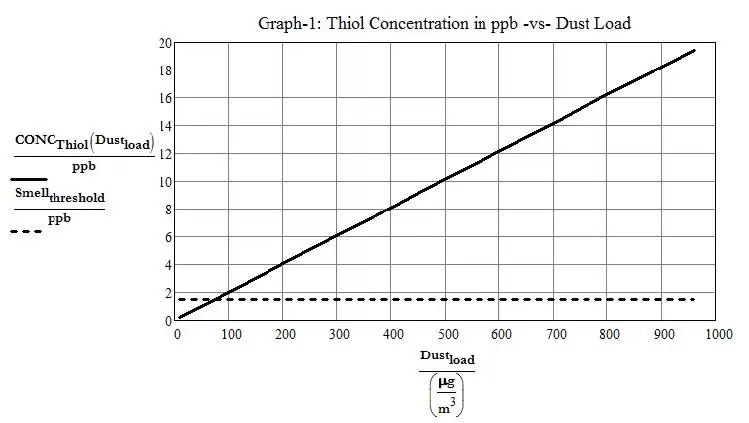
Figure 1. Thiol Concentration in ppb vs. Dust Load
At maximum ASHRAE acceptable airborne dust loads of 10,000 μg/m3 (0.0044 grain/ft3), concentration of thiol could end up being as high as 200 ppb after UV disinfection. According to the US National Institute for Occupational Safety and Health7 (NIOSH), the IDLH (Immediate Danger to Life or Health) level for Methyl Mercaptan is 150 ppm i.e. 150,000 ppb. Also, according to CSST in Quebec as well as OSHA8 (Occupational Safety and Health Administration), the acceptable TLV-TWA (Threshold Limit Value-Time Weighted Average) level for 8hr exposure is 0.5 ppm i.e. 500 ppb. Consequently, the potential levels of thiol concentration generated by UV disinfection are safe even at the highest acceptable airborne dust level.
Given that human occupancy normally generates concentrations of dust well above 75 μg/m3 (0.000033 grain/ft3) and that this dust is mainly made of human dead skin and hair, which consist of keratin and cysteine molecules; and understanding that high energy UV-C photons can break-up these molecules into thiol molecules which have a very low smell threshold, this paper has revealed the root cause of the odor produced by UV disinfection9 of rooms. Given that the resulting potential concentrations of thiol molecules are negligible when compared to the published acceptable exposure limits, it is safe to enter a room after germicidal UV disinfection has been performed.
The authors are grateful to Dr. Wladyslaw Kowalski for data and editorial assistance.
NOMENCLATURE
References